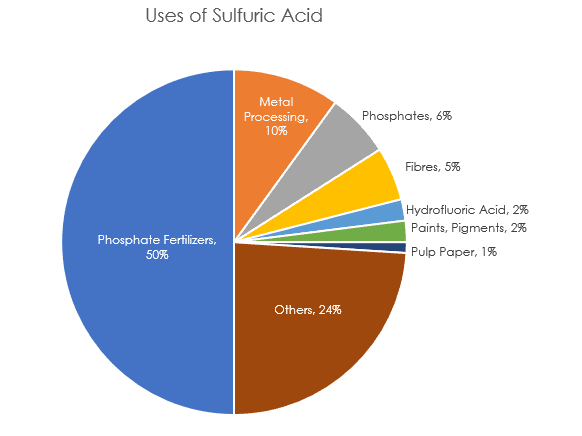
Sulfuric acid (H2SO4) is the most commonly produced and most important industrial chemical in the world because of its use to produce hundreds of other important compounds. One important industrial use of sulfuric acid is in the production of phosphoric acid for the production of fertilizer.
A good catalyst, sulfuric acid is commonly used in the production of esters. It is used in the nitration of toluene and aromatics. Since it is highly reactive with water, it is quite common to see sulfuric acid in a dehydration process.
Learn more about H2SO4 SystemsAnother key application is in petroleum refining, where it is used as a catalyst in the alkylation process of high-octane gasoline production. Today, there are approximately 100 refineries across the U.S. with alkylation units which use either sulfuric acid or hydrofluoric acid as the production catalyst.

Sulfuric acid is a critical component for metal manufacturing, such as the production of copper and zinc and the cleaning of steel. The output of sulfuric acid worldwide grows over 3% a year, partly fueled by growing demand in the Asia-Pacific region.
Because sulfuric acid is used in the manufacturing of important products such as phones, electronics, paper, specialty chemicals, personal care products, and fertilizer, it has an influence on countries’ economies across the world.
A common use of sulfuric acid is to absorb impurities such as metals and organics. After use, there are two choices for handling the spent acid – either neutralize the spent acid and dispose of it or, regenerate it to remove the impurities so the acid can be reused. With the current high demand, sulfuric acid regeneration is quickly becoming the preferred method for both economic and environmental reasons.
Common Processes that require the Regeneration of Sulfuric Acid
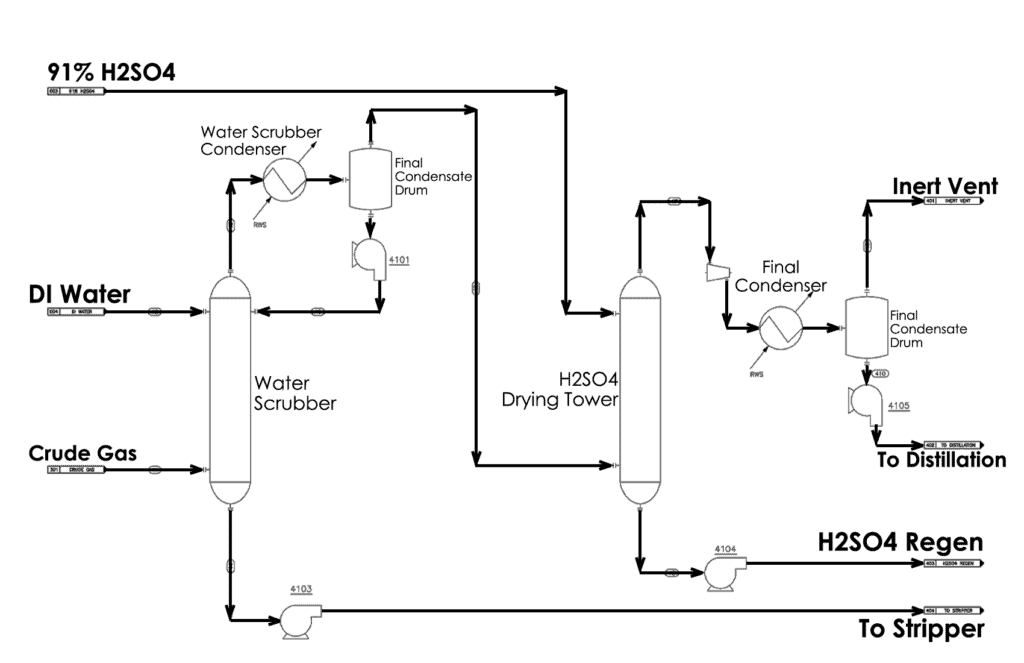
In the alkylation process, H2SO4 catalyst is used to react isobutane with a light olefin to produce the alkylate component used in high octane gasoline. The spent acid contains SO2 that is recovered by cooling it in a process gas tower. This leaves behind lower concentrations of HCl, SO2, and H2SO4. Then, the stream is fed into a sour water stripper where the remaining SO2 is driven off, leaving stripped sour water containing trace levels of H2SO4 and HCl.
The viscose-rayon process uses H2SO4 spin baths in the production of rayon fibers and cellulose films. Both rayon fibers and cellulose have a wide market for clothing, carpet, various plastics, and meat and food casing. In the viscose process, diluted acid is reconcentrated using a graphite evaporator with a lined steel or FRP vapor body followed by a contact condenser. This process takes place under vacuum. Increasing the vacuum results in higher gas volume and increases the level of inerts. CG Thermal can supply expert advice and state-of-the-art systems and equipment to optimize the Capex/Opex for this process.
Because of its affinity for water, a common use of H2SO4 is as a drying agent. For example, it is used to dry chlorine in the production of ethylene dichloride and to provide highly pure AHCl to the chlorine cell membranes in various chloro-alkali processes. The resulting diluted acid is regenerated in a similar manner as in the viscose process described above but at much higher concentration, which presents unique material of construction requirements. Attention must be paid to the process operating temperatures and pressures when material choices are made.
Common Materials of Construction
For heat transfer applications involving sulfuric acid, materials of construction vary depending on the temperature, concentration, impurities, required service life and cost effectiveness. Common cost-effective materials include SiC ceramic, graphite, polyphenylene sulfide (PPS-GR), stainless steels and nickel alloys.
SiC Ceramic
SiC Ceramic is the premium heat transfer material because it is 100% corrosion resistance for all concentrations of sulfuric acid. CG Thermal’s Umax® Advanced Ceramic tube is composed of alpha sintered silicon carbide (SiC) which has no fillers or free silicon, making it universally chemically inert. All metallic material options come with a life expectancy limited by a corrosion rate whereas the Umax advanced ceramic will not corrode.
SiC is 50% harder than tungsten carbide which allows for increased velocities of 9-15 ft/s without limiting tube life due to erosion. The increased flowrate will result in a higher heat transfer rate and reduced fouling rate, thereby reducing the surface area required. Because of SiC’s smooth, impervious surface fouling material will remain entrained in the process fluid as it passes through the tube with little resistance.
Overall, these advantages result in higher thermal efficiency and less required surface area. Though the SiC material is more costly, longevity and reliability make it the material of choice for smaller systems. For large heat exchangers, the operational benefits should be weighed against the cost benefits of other materials.
Graphite
For sulfuric acid concentrations up to 75% and 345°F, Impervite® graphite is the preferred material of choice with optimal CAPEX and OPEX values. It can be considered for concentrations up to 90% under special consideration and with a more limited operating life.

Impervite® graphite is a fully graphitized, more ductile material impregnated with phenolic resin. Its characteristics result from raw material specification, high graphitization temperature which results in a very crystalline graphite structure, and the highly controlled phenolic impregnation process making it a very resilient material with high toughness that will withstand system stresses well beyond the capabilities of carbon-graphite or resin bonded “graphite” tubes.
With metal tubes, corrosion results in thinning of the tube wall. With graphite tubes, this is not the case. However, with impervious graphite tubes in certain conditions (typically higher temperatures and higher acid concentrations), the phenolic resin used to make the tube impervious can be attacked over time. This results in resin loss in the tube and lower tube strength. Such loss of strength can lead to eventual tube breakage. In sulfuric acid concentrations above 85%, the acid can cause the graphite base to oxidize and lead to failure. Still, graphite remains an excellent heat exchanger material for many process applications requiring heating or cooling of sulfuric acid.
PPS-GR

The PPS-GR tube combines the benefits of graphite and polymer materials to provide corrosion resistance, efficiency, and reliability in sulfuric acid processes. This tube material provides an excellent combination of resilience to operating stresses, corrosion resistance, thermal efficiency, low fouling, and maintainability. This is a newer, promising material with success up to 60% concentration at 290°F. CG Thermal is testing this at higher concentrations and continues to see extraordinary results. Even at its current state of verification, this material is a very cost-effective alternative to cathodically protected stainless steel heat exchangers.
Stainless Steel Alloys

In a properly designed unit, SST construction can be used effectively for high-temperature, gas-to-gas recuperators. Recuperators are used in applications such as catalytic operations, high-temperature energy storage, thermal oxidizers, steel mills, and steel foundries. These recuperators are capable of handling temperatures well over 1400°F. However, at the higher temperatures, it is important to understand the capabilities and limitations of the various grades of SST when determining which alloy will maintain the required strength at the design temperatures.
When it comes to liquid process streams, carbon steel and SST are acceptable options for heat transfer materials for concentrations between 93%-99% at room temperature. However, for other concentrations or slightly elevated temperatures, only a handful of specialty alloys can be used if a corrosion rate of less than the customary 5 mpy for heat exchange media is to be maintained. For example, when concentrations exceed 70% at elevated temperatures, tantalum remains the only metallic option.
Transport and Storage
Because of its resistance to corrosion in H2SO4 at all concentrations and most temperatures incurred in regeneration processes, PTFE lined steel is a commonly used for the construction of towers, separation drums, and other vessels utilized in regeneration, especially in positive pressure systems. PTFE can be bonded for use under vacuum using specialized adhesives. However, most adhesives are rated for temperatures less than that of the PTFE lining.
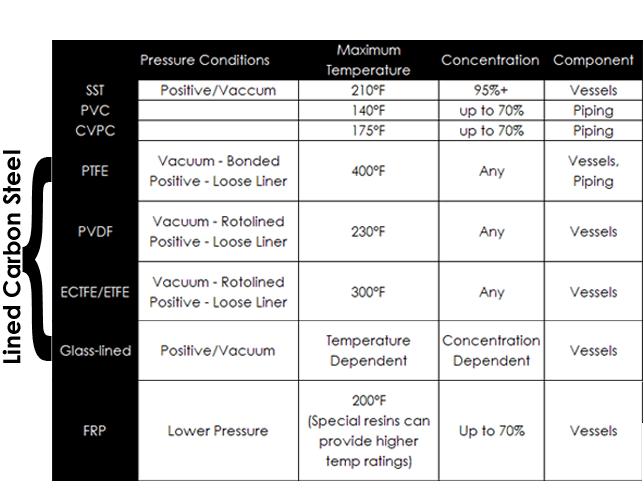
Rotolining offers a mechanically and chemically bonded solution as well as the additional benefit of being seamless. PFA, ECTFE, ETFE, and PVDF can all be applied using the rotolining process. Vessel geometry, design temperature and cost will determine the proper choice of lining material for a given application.
Glass-lined equipment can be a good option for vacuum operation as well. At concentrations near 40%, glass-lining can be used if temperatures are below 300°F. The temperature rating rises with concentration above 40%, and reaches 450°F above 80% concentrations. Therefore, glass-linings are frequently used for vessels in evaporation at higher concentrations.
Fiberglass reinforced polyester (FRP) is a very affordable option and can be used for lower pressure and temperature applications when concentrations are 70% or less. 200°F is the practical temperature limit for FRP, however specialty resins for use at higher temperatures are available. For additional corrosion resistance, the FRP can be lined. This is referred to as dual laminate.
PTFE lined pipe is often used due to its corrosion resistance and temperature rating. CPVC can be used with concentrations less than 70% at temperatures under 175°F.
Design Considerations
Several of sulfuric acid’s thermal properties require particular attention when considering heat and mass transfer systems involved in regeneration.
Viscosity
The viscosity of sulfuric acid varies significantly with temperature and concentration. With increased concentration and a decrease in temperature, viscosity will rapidly increase. For example, viscosity of 5% H2SO4 at 200°F is 0.345cp while that of 93% H2SO4 at 80°F is 17.305cp. This will have a significant impact on pressure drop in the components throughout the system. This effect must be evaluated when designing a system to avoid inflated capital cost and unreasonable operating costs. High viscosity has a significant impact on liquid heat exchangers, pumps, and piping.
An increase in viscosity, µ will result in a decrease in the heat transfer coefficient, h, of the H2SO4 stream since h ~ (Re0.8 )( Pr0.3 ) or (ρνd/µ)0.8 (cρµ/k)0.3 . For instance, a 14-fold increase in viscosity would result in a 4-fold decrease in the heat transfer coefficient of the acid.
Additionally, a lower tube velocity is required with a viscous fluid to keep the pressure drop within an acceptable range. Reduced velocity further reduces the Reynold’s number, Re and in turn the heat transfer coefficient. One solution to consider for reducing pressure drop with high viscosity acid may be to put the acid on the shell side of the heat exchanger using a corrosion resistant shell lining.
Because of these many considerations, a thorough evaluation of the heat exchanger design adds significantly to the value of the overall system design.
Vapor Pressure
Vacuum conditions are frequently required when reconcentrating sulfuric acid because it has a low vapor pressure. At lower concentrations, the vapor pressure is mostly governed by the water. However, at higher concentrations, the degree of vacuum will have a more significant effect on the process temperature, which will in turn affect the energy input for the separation. This could result in higher process temperatures, reduced efficiency, and limited material options.
Electrical Conductivity
Electrical conductivity varies with concentration as a parabolic function, resulting in two concentrations described by the same value. This condition needs to be considered in choosing measurement and control devices. Several approaches can be taken depending on the precision required. A Coriolis meter is commonly used to measure both mass flow and density. This will provide precise measurement and control when low deviations in concentration are required.
CG Thermal has the experience and expertise working with harsh and corrosive materials such as sulfuric acid. To learn more on how we can help improve your competitive position, contact us today.